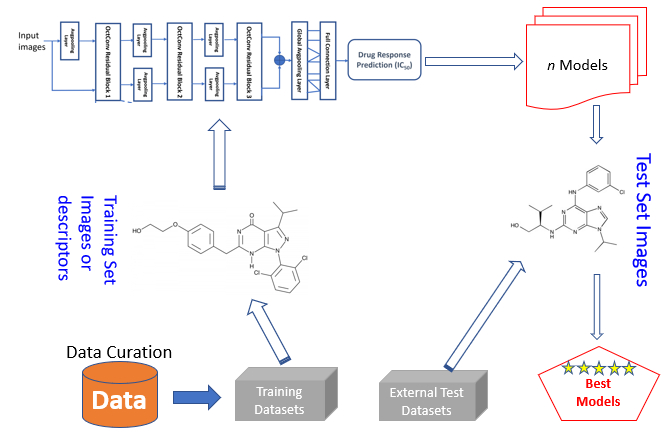
 We have been engaged in development of novel strategies by integrating cheminformatics, bioinformatics, and systems chemical biology approaches (see Software section for specific tools). Our work encompasses curation/analysis of big data, design/development of robust algorithms/workflows, and their application to rational drug discovery. In the area of ligand-based design, we developed new methods such as PolyPharma, JavaDL, iCCBDR, ALL-QSAR, and HERpred. For structure-based design, we implemented miRmo, EnsembleGen, NaMaMeVR, DOVIS, ENTess, PepHemo, etc. Concurrently, we are developing innovative approaches for drug repurposing and polypharmacology studies. Rational design of polypharmacology and repositioning holds great promise for the next generation of drug discovery and development. Using our data and methods, we attempt to identify new indications of existing drugs (drug repurposing), design multi-targeting agents, and guide the use of different drugs for combination therapy. Our goal is to develop a robust and unified e-Research framework by integrating approaches and concepts from different disciplines. As demonstrated, these tools have been used successfully to study many important biological systems and design effective anticancer therapeutics. Relevant publications:
We have been engaged in development of novel strategies by integrating cheminformatics, bioinformatics, and systems chemical biology approaches (see Software section for specific tools). Our work encompasses curation/analysis of big data, design/development of robust algorithms/workflows, and their application to rational drug discovery. In the area of ligand-based design, we developed new methods such as PolyPharma, JavaDL, iCCBDR, ALL-QSAR, and HERpred. For structure-based design, we implemented miRmo, EnsembleGen, NaMaMeVR, DOVIS, ENTess, PepHemo, etc. Concurrently, we are developing innovative approaches for drug repurposing and polypharmacology studies. Rational design of polypharmacology and repositioning holds great promise for the next generation of drug discovery and development. Using our data and methods, we attempt to identify new indications of existing drugs (drug repurposing), design multi-targeting agents, and guide the use of different drugs for combination therapy. Our goal is to develop a robust and unified e-Research framework by integrating approaches and concepts from different disciplines. As demonstrated, these tools have been used successfully to study many important biological systems and design effective anticancer therapeutics. Relevant publications:- Huang B, Fong LWR, Chaudhari R, Zhang S*. Development and evaluation of a java-based deep neural network method for drug response predictions. Front Artif Intell. 2023 Mar 23;6:1069353. DOI: 10.3389/frai.2023.1069353.
- Huang B*, Zhang E, Chaudhari R, Gimperlein H. Sequence-based Optimized Chaos Game Representation and Deep Learning for Peptide/Protein Classification. bioRxiv. 2022.09.10.507145. DOI: 10.1101/2022.09.10.507145.
- Chang AT, Chen L, Song L, Zhang S*, Nikonowicz EP*. 2-Amino-1,3-benzothiazole-6-carboxamide Preferentially Binds the Tandem Mismatch Motif r(UY:GA). Biochemistry. 2020 Sep 8;59(35):3225-3234. DOI: 10.1021/acs.biochem.0c00369.
- Chaudhari R, Fong LW, Tan Z, Huang B, Zhang S*. An up-to-date overview of computational polypharmacology in modern drug discovery. Expert Opin Drug Discov. 2020 May 26:1-20. DOI: 10.1080/17460441.2020.1767063.
2. Develop ubiquitination-based cancer therapies
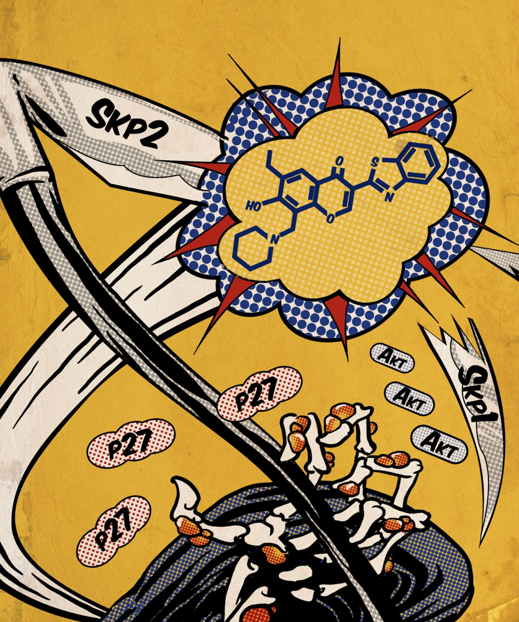 There is a significant therapeutic potential of targeting the protein degradation system. However, current approaches (e.g., proteasome inhibitors) may cause adverse effects due to limited specificity. To address this issue, we attempt to inhibit the specific ubiquitination components that are critical for degradation or signaling. Using our in-house tools and libraries, we have developed first-of-its-kind E3 ubiquitin ligase Skp2 inhibitors. These agents significantly block Skp2 E3 ligase activity and could impair the proliferation of a wide range of cancer cell lines, without affecting the viability of normal cells. Furthermore, these agents suppressed tumor growth in vivo. We demonstrated that the anti-tumorigenic effect was mediated by induction of p53-independent apoptosis and senescence as well as suppression of Akt-driven glycolysis. Intriguingly, they also diminished the accumulation and self-renewal potential of cancer stem cells (CSC). Along the same line, we found that targeting other ubiquitination/deubiquitination proteins, such as VHL and USP15, can also be applied to cancer therapies. These discoveries suggest that targeting ubiquitination is a promising strategy to inhibit cancer progression and overcome cancer resistance or metastasis. Here are some selected publications:
There is a significant therapeutic potential of targeting the protein degradation system. However, current approaches (e.g., proteasome inhibitors) may cause adverse effects due to limited specificity. To address this issue, we attempt to inhibit the specific ubiquitination components that are critical for degradation or signaling. Using our in-house tools and libraries, we have developed first-of-its-kind E3 ubiquitin ligase Skp2 inhibitors. These agents significantly block Skp2 E3 ligase activity and could impair the proliferation of a wide range of cancer cell lines, without affecting the viability of normal cells. Furthermore, these agents suppressed tumor growth in vivo. We demonstrated that the anti-tumorigenic effect was mediated by induction of p53-independent apoptosis and senescence as well as suppression of Akt-driven glycolysis. Intriguingly, they also diminished the accumulation and self-renewal potential of cancer stem cells (CSC). Along the same line, we found that targeting other ubiquitination/deubiquitination proteins, such as VHL and USP15, can also be applied to cancer therapies. These discoveries suggest that targeting ubiquitination is a promising strategy to inhibit cancer progression and overcome cancer resistance or metastasis. Here are some selected publications:- Fong LWR, Lee J, Lin HK, Ueno NT, Zhang S*. A gene signature consisting of ubiquitin ligases and deubiquitinating enzymes of SKP2 is associated with clinical outcome in breast cancer. Sci Rep. 2022 Feb 15;12(1):2478. doi: 10.1038/s41598-022-06451-w. PMCID: PMC8847659.
- Han F, Li CF, Cai Z, Zhang X, Jin G, Zhang WN, Xu C, Wang CY, Morrow J, Zhang S, Xu D, Wang G, Lin HK. The critical role of AMPK in driving Akt activation under stress, tumorigenesis and drug resistance. Nat Commun. 2018 Nov 9;9(1):4728. PMCID: PMC6226490.
- Morrow JK, Lin HK, Sun SC, Zhang S*. Targeting ubiquitination for cancer therapies. Future Med Chem. 2015, 7(17):2333-50. PMCID: PMC4976843.
- Chan CH, Morrow JK, Li CF, Gao Y, Jin G, Moten A, Stagg LJ, Ladbury JE, Cai Z, Xu D, Logothetis CJ, Hung MC, Zhang S*, Lin HK*. Pharmacological Inactivation of Skp2 SCF Ubiquitin Ligase Restricts Cancer Stem Cell Traits and Cancer Progression. Cell. 2013, 154(3):556-68. PMCID: PMC3845452.
3. Target traditionally viewed "undruggable" pathways
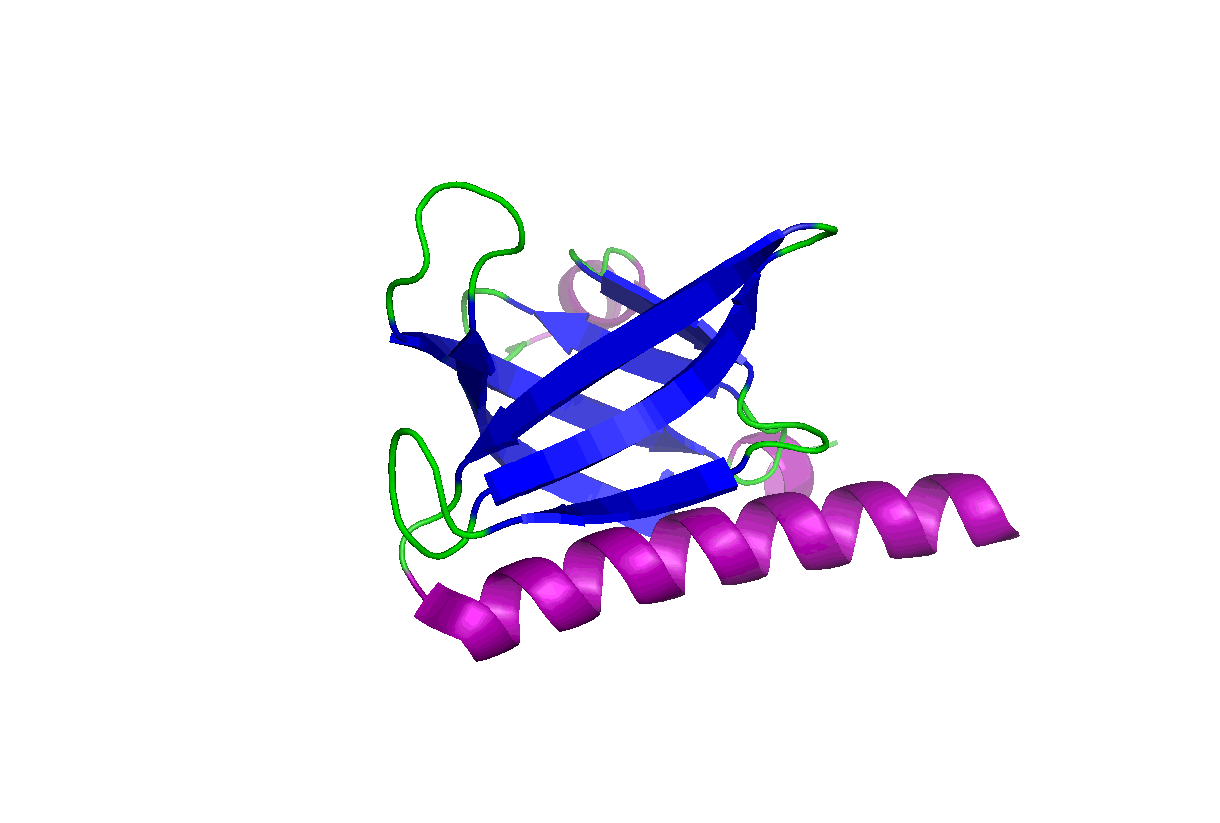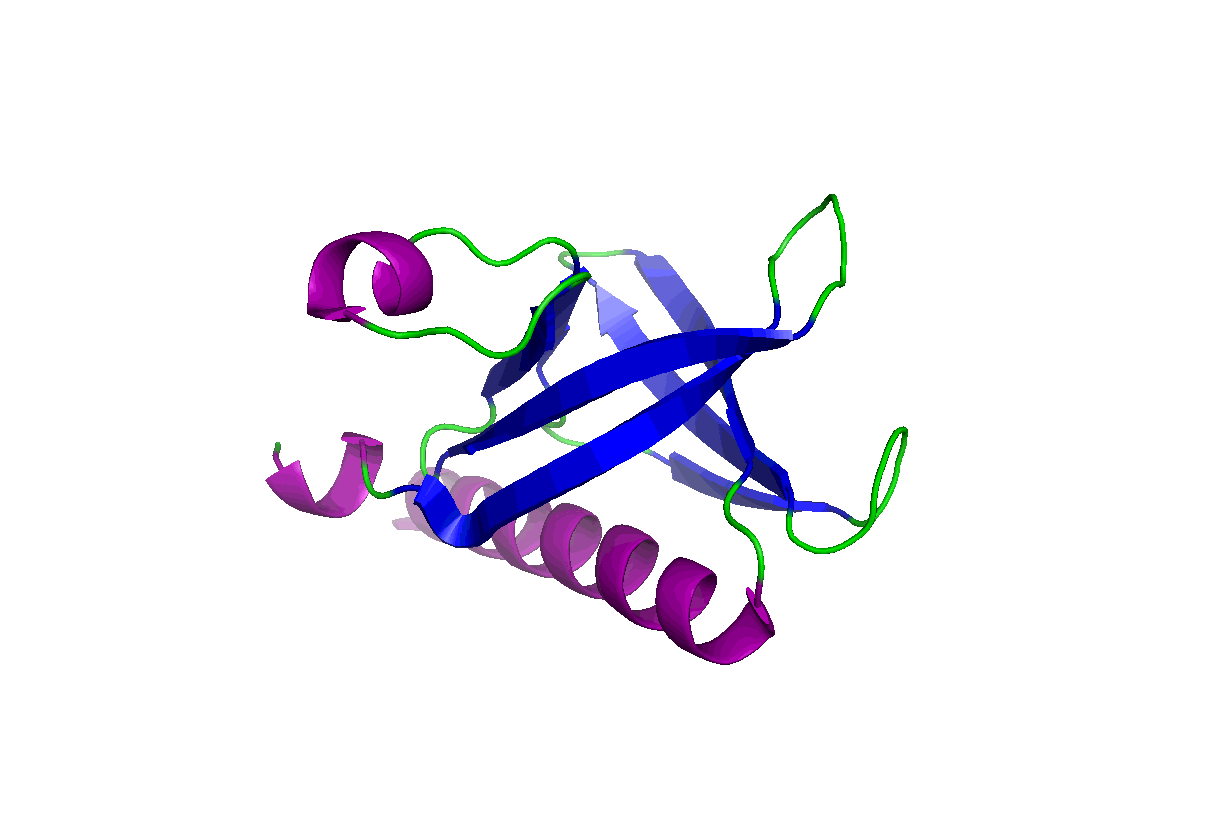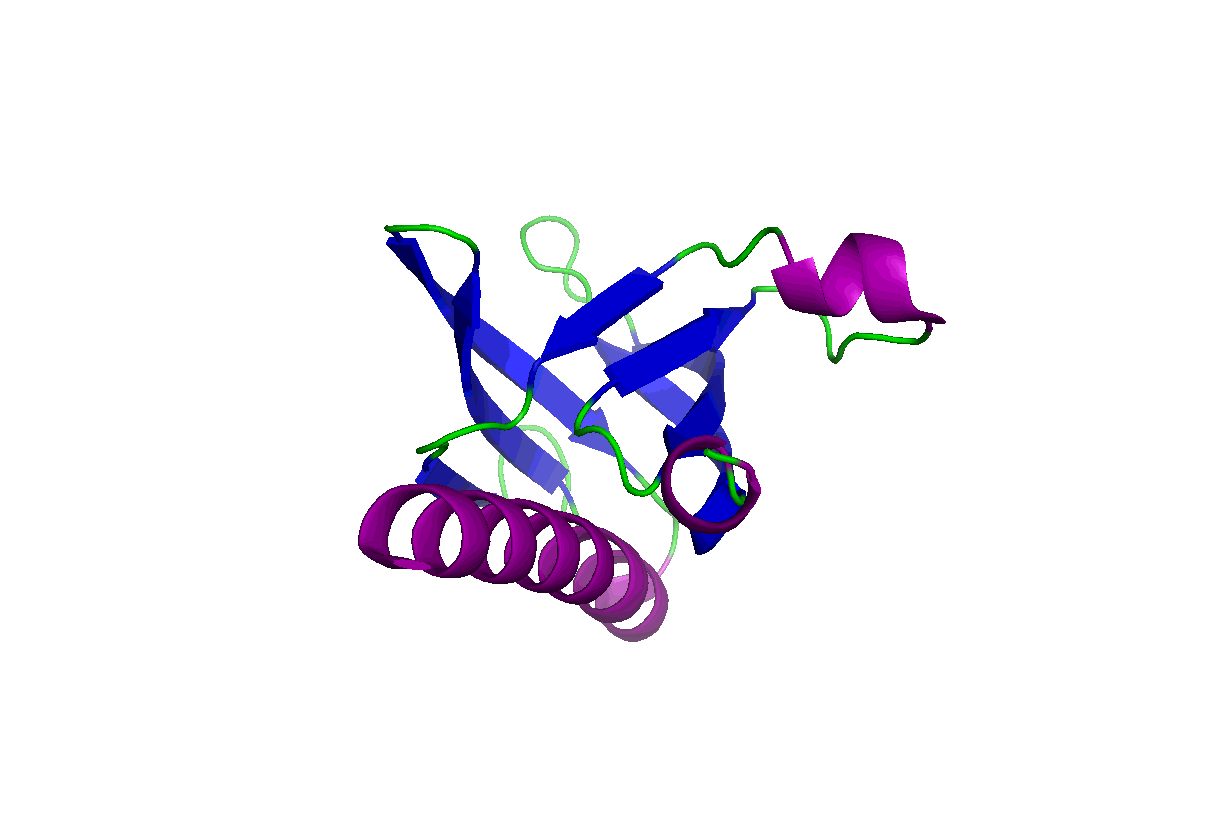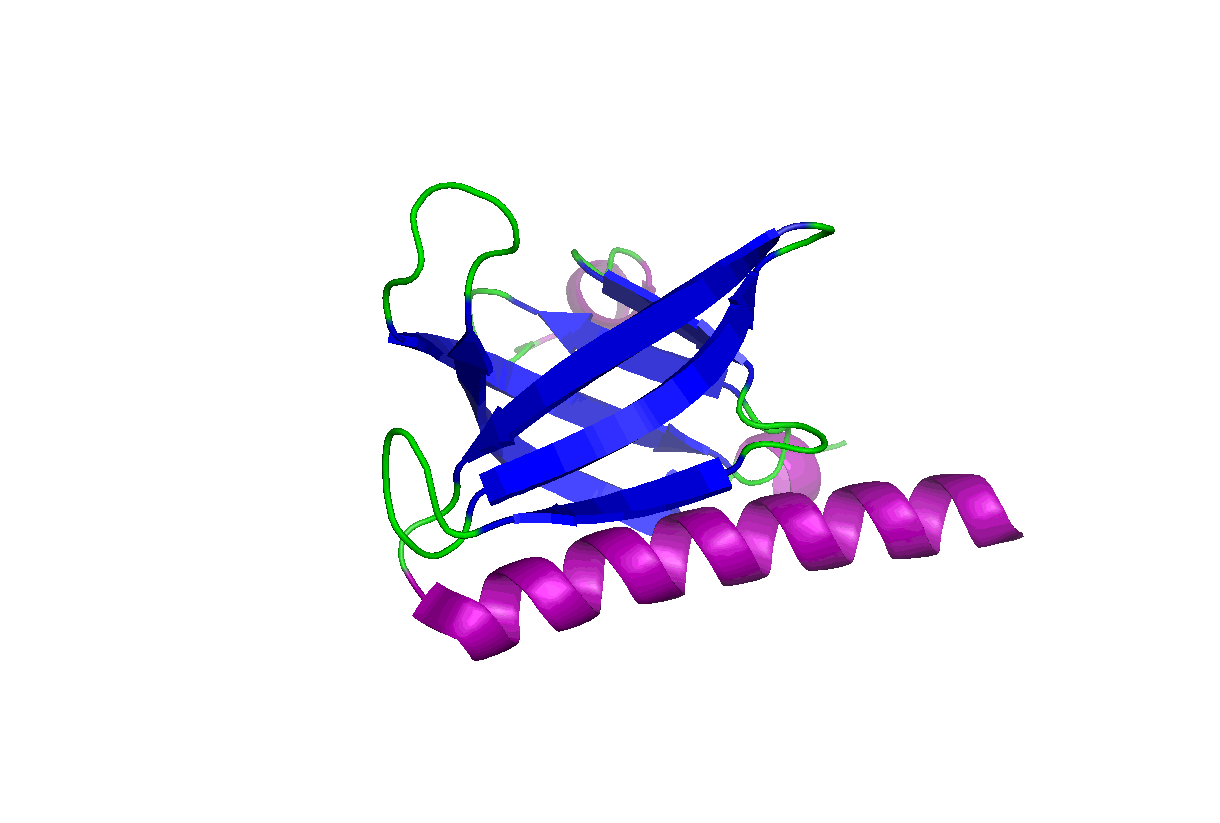 These studies will change our view of target druggability and dramatically expand the target space for drug discovery and development. In particular, we have been studying the pleckstrin homology (PH) domains using our unique computation and experiment-integrated platform. By targeting the Akt and PDK-1 PH domains, we developed novel potent dual inhibitors that were successfully licensed for further clinical studies and commercial development. We have also been involved in mechanistic studies of KRas, Hippo-YAP, GAB1, Caspase, etc. Some relevant publications:
These studies will change our view of target druggability and dramatically expand the target space for drug discovery and development. In particular, we have been studying the pleckstrin homology (PH) domains using our unique computation and experiment-integrated platform. By targeting the Akt and PDK-1 PH domains, we developed novel potent dual inhibitors that were successfully licensed for further clinical studies and commercial development. We have also been involved in mechanistic studies of KRas, Hippo-YAP, GAB1, Caspase, etc. Some relevant publications: - Zhang L, Qu J, Qi Y, Duan Y, Huang YW, Zhou Z, Li P, Yao J, Huang B, Zhang S, Yu D. EZH2 engages TGFβ signaling to promote breast cancer bone metastasis via integrin β1-FAK activation. Nat Commun. 2022 May 10;13(1):2543. doi: 10.1038/s41467-022-30105-0. PMCID: PMC9091212.
- Yao W, Rose JL, Wang W, Seth S, Jiang H, Taguchi A, Liu J, Yan L, Kapoor A, Hou P, Chen Z, Wang Q, Nezi L, Xu Z, Yao J, Hu B, Pettazzoni PF, Ho IL, Feng N, Ramamoorthy V, Jiang S, Deng P, Ma GJ, Den P, Tan Z, Zhang S, et al. Syndecan1 is a critical mediator of macropinocytosis in pancreatic cancer. Nature. 2019. 568(7752):410-414. PMCID: PMC6661074.
- Li N, Wang Y, Neri S, Zhen Y, Fong LWR, Qiao Y, Li X, Chen Z, Stephan C, Deng W, Ye R, Jiang W, Zhang S, et al. Tankyrase disrupts metabolic homeostasis and promotes tumorigenesis by inhibiting LKB1-AMPK signalling. Nat Commun. 2019, 10(1):4363. PMCID: PMC6761205.
- Chen L, Moses SA, Du-Cuny L, Mathew S, Dumas S, Song Z, Meuillet EJ, Zhang S*. Identification and evaluation of potent small molecule inhibitors targeting GAB1 pleckstrin homology domain. PLoS Comput. Biol. 2015 Jan 8;11(1):e1004021. PMCID: PMC4287437.
4. Conduct drug repurposing and predictive ADMET modeling
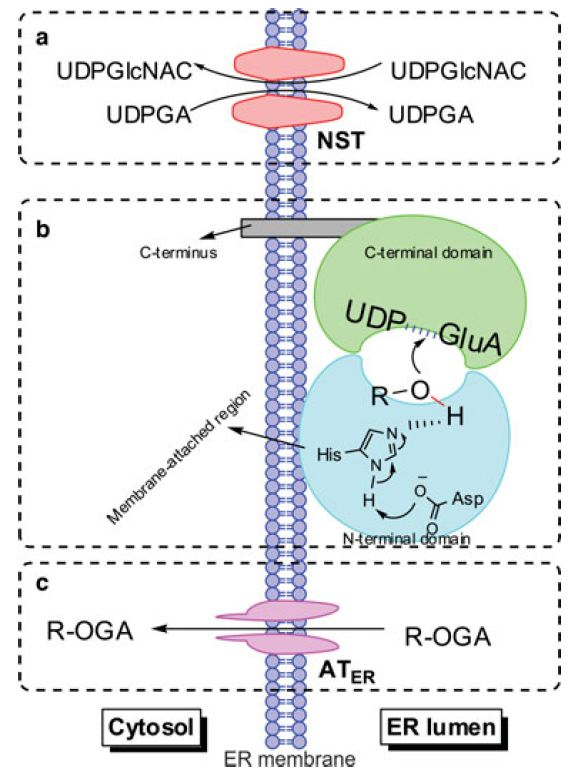 Predictive ADMET is the new 'hip' area in drug discovery. The aim is to build models that link structural changes with changes in response, from which compounds with improved properties can be designed. I have been credited for development of predictive models for several end points including drug permeability, plasm protein binding, hERG inhibition, etc. In particular, we have been interested in studying drug Phase II metabolism by enzymes such as UGT and SULT. We are attempting to build ligand/structure-based models to predict drug metabolism by these enzymes and understand how to use the specific functions/localization of these enzymes to improve the therapeutic efficacy of drugs that have severe side effects or limited bioavailability. Relevant publications:
Predictive ADMET is the new 'hip' area in drug discovery. The aim is to build models that link structural changes with changes in response, from which compounds with improved properties can be designed. I have been credited for development of predictive models for several end points including drug permeability, plasm protein binding, hERG inhibition, etc. In particular, we have been interested in studying drug Phase II metabolism by enzymes such as UGT and SULT. We are attempting to build ligand/structure-based models to predict drug metabolism by these enzymes and understand how to use the specific functions/localization of these enzymes to improve the therapeutic efficacy of drugs that have severe side effects or limited bioavailability. Relevant publications:- Robichaux JP, Elamin YY, Tan Z, Carter BW, Zhang S, Liu S, Li S, Chen T, Poteete A, Estrada-Bernal A, Le AT, Truini A, Nilsson MB, Sun H, Roarty E, Goldberg SB, Brahmer JR, Altan M, Lu C, Papadimitrakopoulou V, Politi K, Doebele RC, Wong KK, Heymach JV. Mechanisms and clinical activity of an EGFR/HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer. Nat Med. 2018. May;24(5):638-646. PMCID: PMC5964608.
- Alla SR, Tan Z, Zhang S*. Curation and Analysis of Multi-targeting Agents for Polypharmacological Modeling. J. Chem. Inf. Model. 2014, 54(9): 2536-43. PMCID: PMC4170814.
- Wu B, Dong D, Hu M, Zhang S*. Quantitative Prediction of Glucuronidation in Humans Using the In Vitro-In Vivo Extrapolation Approach. Curr Top Med Chem. 2013, 13(11):1343-52. DOI: 10.2174/15680266113139990038.
- Du-Cuny L, Chen L, Zhang S*. A Critical Assessment of Combined Ligand/Structure-based Approaches to hERG Channel Blocker Modeling. J Chem Inf Model. 2011, 51(11):2948-2960. PMCID: PMC3894065.
5. Develop novel technologies for non-coding RNA studies
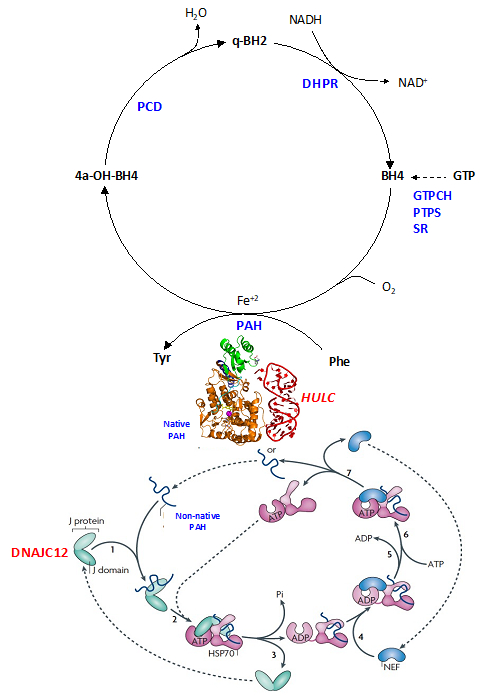 We embarked among the first groups to develop an integrated platform for miRNA modeling by incorporating computational approaches and direct structural studies. Due to its intrinsic challenges, RNA modeling is still largely an unchartered area compared to protein modeling. However, miRNAs are crucial in many disease development and progression. Therefore, the impact of this work is tremendous and we are combining in silico and NMR/crystallography methods to develop an iterative computational/experimental workflow to predict 3D structures of miRNAs, identify novel miRNA-protein interactions and elucidate their disease implications, and design novel agents to experimentally probe miRNA functions and target miRNAs for therapies. We have published several important papers with support from NIH, DOD and NSF:
We embarked among the first groups to develop an integrated platform for miRNA modeling by incorporating computational approaches and direct structural studies. Due to its intrinsic challenges, RNA modeling is still largely an unchartered area compared to protein modeling. However, miRNAs are crucial in many disease development and progression. Therefore, the impact of this work is tremendous and we are combining in silico and NMR/crystallography methods to develop an iterative computational/experimental workflow to predict 3D structures of miRNAs, identify novel miRNA-protein interactions and elucidate their disease implications, and design novel agents to experimentally probe miRNA functions and target miRNAs for therapies. We have published several important papers with support from NIH, DOD and NSF:- Li Y, Tan Z, Zhang Y, Zhang Z, Hu Q, Liang K, Jun Y, Ye Y, Li YC, Li C, Liao L, Xu J, Xing Z, Pan Y, Chatterjee SS, Nguyen TK, Hsiao H, Egranov SD, Putluri N, Coarfa C, Hawke DH, Gunaratne PH, Tsai KL, Han L, Hung MC, Calin GA, Namour F, Guéant JL, Muntau AC, Blau N, Sutton VR, Schiff M, Feillet F*, Zhang S*, Lin C*, Yang L*. A noncoding RNA modulator potentiates phenylalanine metabolism in mice. Science. 2021 Aug 6;373(6555):662-673. DOI: 10.1126/science.aba4991.
- Amero P, Lokesh GLR, Chaudhari RR, Cardenas-Zuniga R, Schubert T, Attia YM, Montalvo-Gonzalez E, Elsayed AM, Ivan C, Wang Z, Cristini V, Franciscis V, Zhang S, et al. Conversion of RNA Aptamer into Modified DNA Aptamers Provides for Prolonged Stability and Enhanced Antitumor Activity. J Am Chem Soc. 2021 May 26;143(20):7655-7670. DOI: 10.1021/jacs.9b10460. Epub 2021 May 14.
- Li C, Wang S, Xing Z, Lin A, Liang K, Song J, Hu Q, Yao J, Chen Z, Park PK, Hawke DH, Zhou J, Zhou Y, Zhang S, et al. A ROR1-HER3-LncRNA signaling axis modulates the Hippo-YAP pathway to regulate bone metastasis. Nat. Cell Biol. 2017,19(2):106-119. PMCID: PMC5336186.
- Chen L, Calin GA, Zhang S*. Novel insights of structure-based modeling for RNA-targeted drug discovery. J Chem Inf Model. 2012 Oct 22;52(10):2741-53. doi: 10.1021/ci300320t. Epub 2012 Sep 21. PMCID: PMC3869234.
